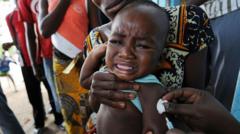
A recent US-funded vaccine trial in Guinea-Bissau has come under fire from the World Health Organization. The trial, which aims to test the efficacy of a new vaccine on infants, has raised concerns over its methodology and potential risks to the participants. Health experts have expressed outrage over the trial, citing lack of transparency and inadequate informed consent from the parents of the infants involved. The trial has sparked a heated debate over the ethics of medical research in developing countries. Critics argue that such trials often exploit vulnerable populations and disregard local health regulations. The WHO has called for an immediate halt to the trial, pending a thorough investigation into the allegations. The incident has raised questions over the role of international organizations in regulating medical research and protecting human rights. As the controversy continues to unfold, it remains to be seen how the situation will be resolved. The US government has yet to comment on the issue, fueling further speculation and criticism. The international community is watching closely, awaiting a response from the authorities involved. The Guinea-Bissau government has announced an investigation into the matter, but the outcome is still uncertain.
Keywords: vaccine trial, US funded, Guinea-Bissau, World Health Organization, medical research, ethics, human rights, informed consent, transparency, exploitation, developing countries, health regulations, international organizations, US government
Source: BBC

%20(5).png)



.png)
